Carbon Dioxide Capture and Sequestration: Overview
On This Page
- What is carbon dioxide capture and sequestration?
- Why is it important?
- What sources of carbon dioxide can it be applied to?
- Where can captured carbon dioxide be stored?
Featured Topics
What is carbon dioxide capture and sequestration?
Carbon dioxide (CO2) capture and sequestration (CCS) is a set of technologies that can greatly reduce CO2 emissions from new and existing coal- and gas-fired power plants and large industrial sources. CCS is a three-step process that includes:
- Capture of CO2 from power plants or industrial processes
- Transport of the captured and compressed CO2 (usually in pipelines).
- Underground injection and geologic sequestration (also referred to as storage) of the CO2 into deep underground rock formations. These formations are often a mile or more beneath the surface and consist of porous rock that holds the CO2. Overlying these formations are impermeable, non-porous layers of rock that trap the CO2 and prevent it from migrating upward.
The figure below illustrates the general CCS process and shows a typical depth at which CO2 would be injected.
CCS Schematic (Subsurface depth to scale, 5,280 feet equals one mile)
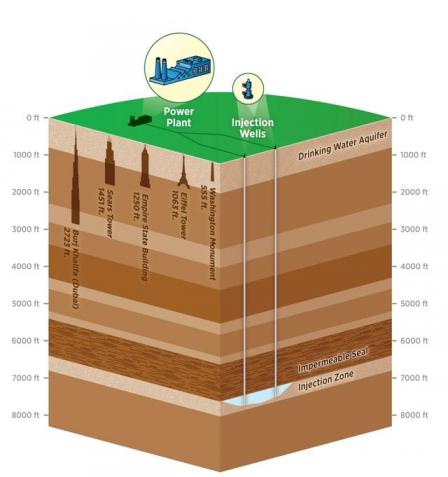
Why is it important?
Carbon dioxide (CO2) capture and sequestration (CCS) could play an important role in reducing greenhouse gas emissions, while enabling low-carbon electricity generation from power plants. As estimated in the U.S. Inventory of Greenhouse Gas Emissions and Sinks, more than 40% of CO2 emissions in the United States are from electric power generation. CCS technologies are currently available and can dramatically reduce (by 80-90%) CO2 emissions from power plants that burn fossil fuels. Applied to a 500 MW coal-fired power plant, which emits roughly 3 million tons of CO2 per year,[1] the amount of GHG emissions avoided (with a 90% reduction efficiency) would be equivalent to:
- Planting more than 62 million trees, and waiting at least 10 years for them to grow.
- Avoiding annual electricity-related emissions from more than 300,000 homes.
To see this emissions reduction expressed in other equivalent terms, see EPA's Carbon Equivalencies Calculator.
CCS could also viably be used to reduce emissions from industrial process such as cement production and natural gas processing facilities.
What sources of carbon dioxide can it be applied to?
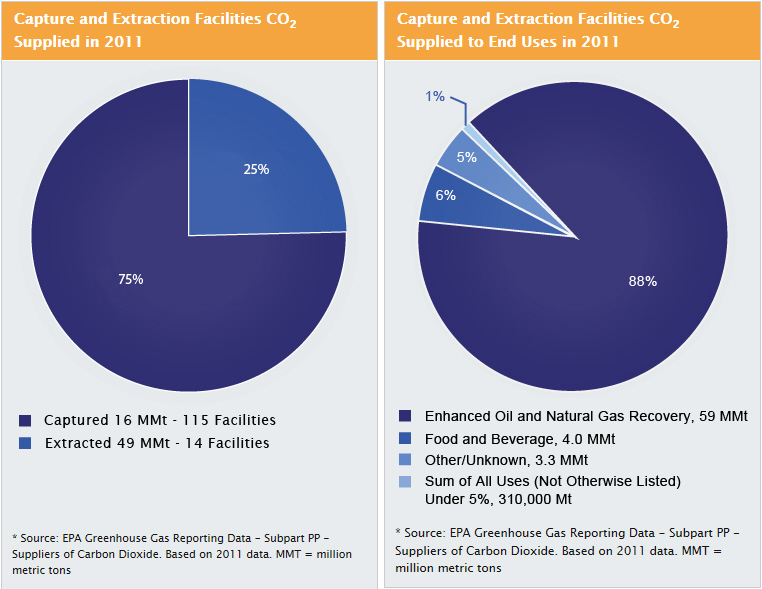
Carbon dioxide (CO2) capture and sequestration (CCS) can significantly reduce emissions from large stationary sources of CO2, which include coal- and natural-gas-fired power plants, as well as certain industry types such as ethanol and natural gas processing plants. EPA's Greenhouse Gas Reporting Program includes facilities that capture CO2 for the purpose of supplying the CO2 to the economy or for injecting it underground (Subpart PP). According to the Greenhouse Gas Reporting Program, CO2 capture is currently occurring at over 120 facilities in the United States, mainly on industrial processes, and the CO2 is used for a wide range of end uses. End uses of CO2 include enhanced oil recovery (EOR), food and beverage manufacturing, pulp and paper manufacturing, and metal fabrication. The figure below shows the portion of CO2 that is currently being captured from power plants and other industrial facilities and the portion that is extracted by production wells from natural CO2 bearing formations in the United States. The second figure shows the various domestic end uses of captured and extracted CO2. (Note that natural sources of CO2 are not considered in the Total CO2 Supply End Uses figure). As CCS becomes more widespread, it is expected that the portion of CO2 captured in the United States from power generation and industrial processes will increase.
Where can captured carbon dioxide be stored?
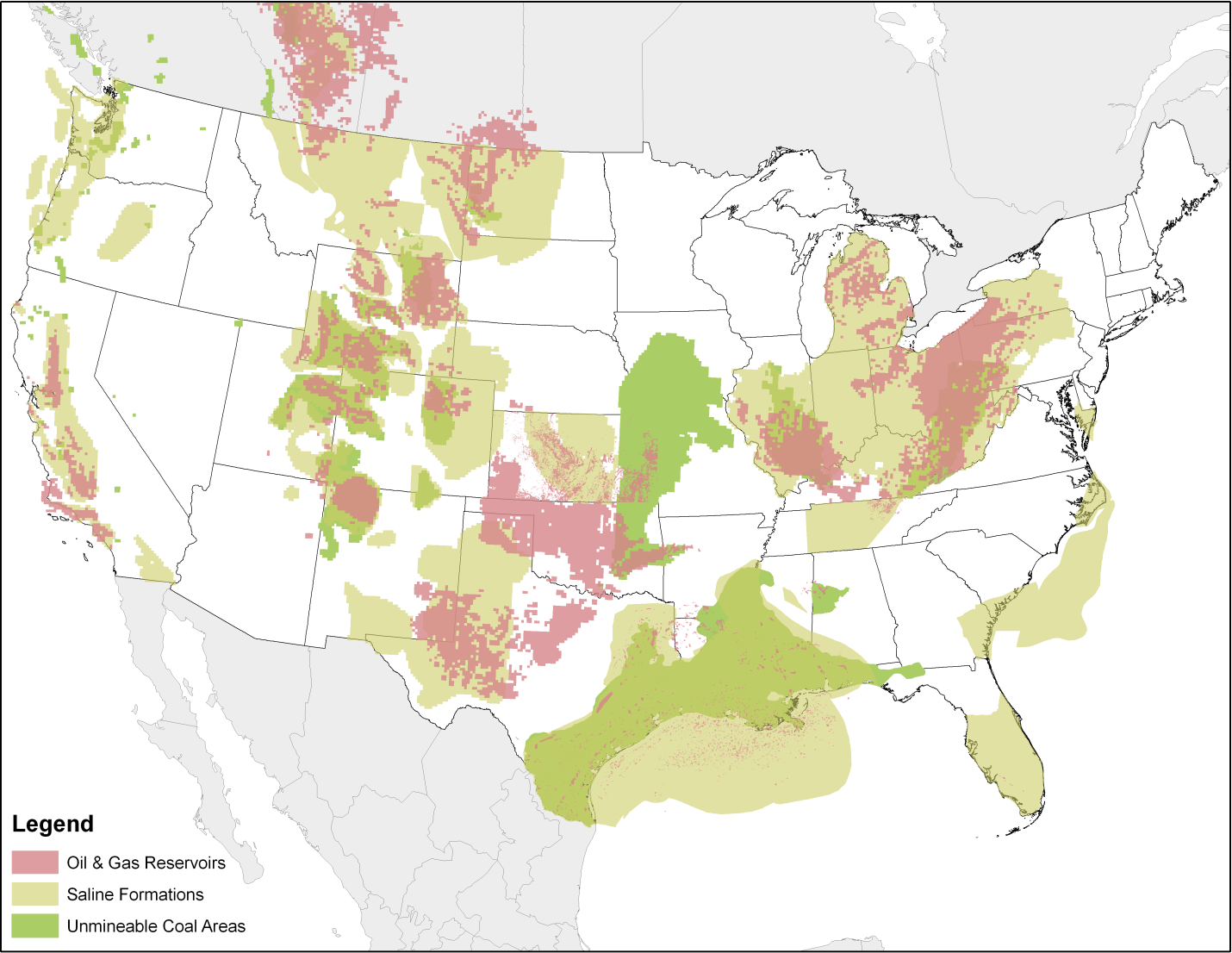
After capture, carbon dioxide (CO2) is compressed and then transported to a site where it is injected underground for permanent storage (also known as "sequestration"). CO2 is commonly transported by pipeline, but it can also be transported by train, truck, or ship. Geologic formations suitable for sequestration include depleted oil and gas fields, deep coal seams, and saline formations. The U.S. Department of Energy estimates that anywhere from 1,800 to 20,000 billion metric tons of CO2 could be stored underground in the United States.[2] That is equivalent to 600 to 6,700 years of current level emissions from large stationary sources in the United States.[3]
Overview of Geologic Storage Potential in the United States (Source: U.S. Department of Energy, NATCARB)
Potential sequestration sites must undergo appropriate site characterization to ensure that the site can safely and securely store CO2. After being transported to the sequestration site, the compressed CO2 is injected deep underground into solid, but porous rock, such as sandstone, shale, dolomite, basalt, or deep coal seams. Suitable formations for CO2 sequestration are located under one or more layers of cap rock, which trap the CO2 and prevent upward migration. These sites are then rigorously monitored to ensure that the CO2 remains permanently underground. The safety and security of CO2 geologic sequestration is a priority for EPA.
For more information, visit the National Carbon Sequestration Database and Geographic Information System (NATCARB), Exit a geographic information system (GIS)-based tool developed to provide an overview of CCS projects and storage potential.
References
[1] MIT (2007) The Future of Coal: Options for a Carbon-Constrained World, Exit Massachusetts Institute of Technology, 2007.
[2] NACAP (2012) The North American Carbon Storage Atlas, Exit The U.S. Department of Energy (DOE), Natural Resources Canada (NRCan), and the Mexican Ministry of Energy (SENER).
[3] GHGRP (2012) EPA Greenhouse Gas Reporting Data-Subpart PP-Suppliers of Carbon Dioxide. Based on 2011 data.
